Share
Calculate the mass of water produced when 1.57g of butane reacts with excess oxygen
Question
Lost your password? Please enter your email address. You will receive a link and will create a new password via email.
Answers ( )
Explanation:
So, first you will want to write the balanced chemical equation for this reaction.
Butane =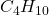
^ This ends up being your balanced chemical equation. Now, you can do the math!
After plugging this into a calculator, your final mass of water should be:
2.43gH2O