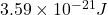Share
Boltzmann’s constant is 1.38066×10⁻²³ J/K, and the universal gas constant is 8.31451 J/K · mol. If 3 mol of a gas is confined to a 6.1 L ves
Question
Boltzmann’s constant is 1.38066×10⁻²³ J/K, and the universal gas constant is 8.31451 J/K · mol. If 3 mol of a gas is confined to a 6.1 L vessel at a pressure of 7 atm, what is the average kinetic energy of a gas molecule? Answer in units of J.
in progress
0
Physics
4 years
2021-07-28T14:45:38+00:00
2021-07-28T14:45:38+00:00 1 Answers
14 views
0

Answers ( )
Answer:
Explanation:
We are given that
Boltzmann’s constant,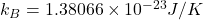
Universal gas constant,R=8.31451 J/K
Number of moles,n=3
Volume ,V=6.1 L=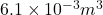
Pressure,P=7 atm=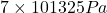
T=173.45 K
Average kinetic energy=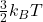
Average kinetic energy=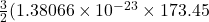
Average kinetic energy=