Share
An ionic bond is a bond that forms between Olons with opposite charges. O Atoms with neutral charges. O One atom’s nucleus
Question
Lost your password? Please enter your email address. You will receive a link and will create a new password via email.
Answers ( )
Answer: An ionic bond is a bond that forms between ions with opposite charges.
Explanation:
A chemical bond formed by the transfer of electrons from one atom to another is called an ionic bond.
For example, sodium has electronic distribution as 2, 8, 1 and chlorine has electronic distribution as 2, 8, 7.
In order to attain stability, sodium needs to lose 1 electron and chlorine needs to gain one electron. Therefore, sodium will transfer its one valence electron (forming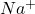 ion) to chlorine atom (forming
ion) to chlorine atom (forming 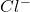 ion) which leads to the formation of NaCl compound.
ion) which leads to the formation of NaCl compound.
Thus, we can conclude that an ionic bond is a bond that forms between ions with opposite charges.