Share
Aluminum and cloride undergoes a synthesis reaction if 8molnof Al reacts with 10mol of cl, what is the maximum amount of AlCl3 can produce
Question
Lost your password? Please enter your email address. You will receive a link and will create a new password via email.
Answers ( )
Answer: The mass of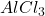 produced is 889.38 g
produced is 889.38 g
Explanation:
We are given:
Moles of Al = 8 mol
Moles of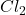 = 10 mol
= 10 mol
For the given chemical reaction:
By stoichiometry of the reaction:
If 3 moles of chlorine gas reacts with 2 moles of Al
So, 10 moles of chlorine gas will react with =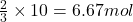 of Al
of Al
As the given amount of Al is more than the required amount. Thus, it is present in excess and is considered as an excess reagent.
Thus, chlorine gas is considered a limiting reagent because it limits the formation of the product.
By the stoichiometry of the reaction:
If 3 moles of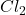 produces 2 mole of
produces 2 mole of 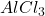
So, 10 moles of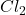 will produce =
will produce = 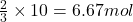 of
of 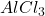
The number of moles is defined as the ratio of the mass of a substance to its molar mass. The equation used is:
We know, molar mass of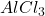 = 133.34 g/mol
= 133.34 g/mol
Putting values in above equation, we get:
Hence, the mass of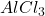 produced is 889.38 g
produced is 889.38 g