Share
A sealed syringe contains 10 × 10−6 m3 of air at 1 × 105 Pa. The plunger is pushed until the volume of trapped air is 4 × 10−6 m3. If there
Question
A sealed syringe contains 10 × 10−6 m3 of air at 1 × 105 Pa. The plunger is pushed until the volume of trapped air is 4 × 10−6 m3. If there is no change in temperature, what is the new pressure of the gas?
in progress
0
Physics
4 years
2021-07-28T08:42:47+00:00
2021-07-28T08:42:47+00:00 1 Answers
94 views
0

Answers ( )
Answer: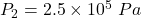
Explanation:
Given
Initial volume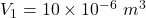
Initial Pressure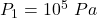
Final trapped air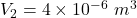
If there is no change in temperature then We can write