Share
A quantity of gas with an initial volume of 5 cubic feet and a pressure of 1700 pounds per square foot expands to a volume of 9 cubic feet.
Question
A quantity of gas with an initial volume of 5 cubic feet and a pressure of 1700 pounds per square foot expands to a volume of 9 cubic feet. Find the work done by the gas for the given volume and pressure. Round your answer to two decimal places. Assume the temperature of the gas in this process remain constant.
in progress
0
Physics
3 years
2021-07-16T19:39:56+00:00
2021-07-16T19:39:56+00:00 1 Answers
8 views
0

Answers ( )
Answer:
Work done by the gas for the given volume and pressure pounds foot
pounds foot
Explanation:
Given
Pressure applied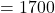 pounds per square foot
pounds per square foot
Initial Volume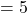 cubic feet
cubic feet
Final Volume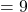 cubic feet
cubic feet
As we know pressure is inversely proportional to V
where k is the proportionality constant
V is the volume and
P is the pressure
Work done
Integrating the above equation, we get-
Work done by the gas for the given volume and pressure pounds foot
pounds foot