Share
A metal forms the fluoride MF3. Electrolysis of the molten fluoride by a current of 3.86 A for 16.2 minutes deposits 1.25 g of the metal. Ca
Question
Lost your password? Please enter your email address. You will receive a link and will create a new password via email.
Answers ( )
Answer: The molar mass of the metal is 96.45 g/mol
Explanation:
The fluoride of the metal formed is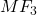
The oxidation half-reaction follows:
Calculating the theoretical mass deposited by using Faraday’s law, which is:
where,
m = actual mass deposited = 1.25 g
M = molar mass of metal = ?
I = average current = 3.86 A
t = time period in seconds = 16.2 min = 972 s (Conversion factor: 1 min = 60 sec)
n = number of electrons exchanged =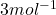
F = Faraday’s constant = 96500 C
Putting values in equation 1, we get:
Hence, the molar mass of the metal is 96.45 g/mol