Share
A doubly ionized molecule (i.e., a molecule lacking two electrons) moving in a magnetic field experiences a magnetic force of 6.75 × 10 − 16
Question
A doubly ionized molecule (i.e., a molecule lacking two electrons) moving in a magnetic field experiences a magnetic force of 6.75 × 10 − 16 N 6.75×10−16 N as it moves at 207 m/s 207 m/s at 74.9 ∘ 74.9∘ to the direction of the field. Find the magnitude of the magnetic field.
in progress
0
Physics
3 years
2021-07-27T12:49:03+00:00
2021-07-27T12:49:03+00:00 1 Answers
20 views
0

Answers ( )
Answer:
The magnitude of the magnetic field is 10.19 T.
Explanation:
Given that,
Charge on the doubly charged molecule, q = 2e
Magnetic force,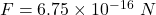
Speed of molecule, v = 207 m/s
The angle to the direction of the field is 74.9 degrees.
We need to find the magnitude of magnetic field. The formula of magnetic force is given by :
B is magnetic field
So, the magnitude of the magnetic field is 10.19 T.