Share
A cylinder contains 3.5 L of oxygen at 350 K and 2.7 atm . The gas is heated, causing a piston in the cylinder to move outward. The heating
Question
A cylinder contains 3.5 L of oxygen at 350 K and 2.7 atm . The gas is heated, causing a piston in the cylinder to move outward. The heating causes the temperature to rise to 620 K and the volume of the cylinder to increase to 9.1 L.What is the gas pressure? P= _____atm
in progress
0
Physics
4 years
2021-08-06T22:11:36+00:00
2021-08-06T22:11:36+00:00 1 Answers
36 views
0

Answers ( )
Answer:
The pressure is
Explanation:
From the question we are told that
The first volume of is
The first pressure is
The first temperature is
The new temperature is
The new volume is
Generally according to the combined gas law we have that
=>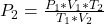
=>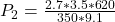
=>