Share
A 2.50-kg steel gasoline can holds 20.0 L of gasoline when full. What is the average density of the full gas can, taking into account the vo
Question
Lost your password? Please enter your email address. You will receive a link and will create a new password via email.
Answers ( )
Answer:
The average density of the full gas can is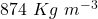 , assuming the standard density of gasoline to be
, assuming the standard density of gasoline to be 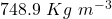 .
.
Explanation:
Given the mass of the can (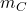 ) is 2.5 Kg and the volume (V) occupied by gasoline is 20 L. Assuming the standard density of gasoline (
) is 2.5 Kg and the volume (V) occupied by gasoline is 20 L. Assuming the standard density of gasoline (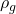 ) to be 748.9
) to be 748.9 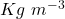 , if ‘
, if ‘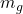 ‘ be the mass of the gasoline occupied in the entire can, then
‘ be the mass of the gasoline occupied in the entire can, then
If ‘ ‘ be the average density of the full gas can, then
‘ be the average density of the full gas can, then