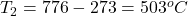Share
A Jaguar XK8 convertible has an eight-cylinder engine. At the beginning of its compression stroke, one of the cylinders contains 499 cm3cm3
Question
A Jaguar XK8 convertible has an eight-cylinder engine. At the beginning of its compression stroke, one of the cylinders contains 499 cm3cm3 of air at atmospheric pressure (1.01×1051.01×105 PaPa) and a temperature of 27.0∘C∘C. At the end of the stroke, the air has been compressed to a volume of 46.2 cm3cm3 and the gauge pressure has increased to 2.72×1062.72×106 PaPa.
in progress
0
Physics
4 years
2021-08-17T06:58:22+00:00
2021-08-17T06:58:22+00:00 1 Answers
336 views
0

Answers ( )
Complete Question
A Jaguar XK8 convertible has an eight-cylinder engine. At the beginning of its compression stroke, one of the cylinders contains 499cm3 of air at atmospheric pressure (1.01×105Pa) and a temperature of 27.0∘C. At the end of the stroke, the air has been compressed to a volume of 46.2cm3 and the gauge pressure has increased to 2.72×106Pa. Compute the final temperature.
Answer:
The final temperature is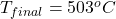
Explanation:
From the question we are given
The volume of one of the cylinders is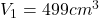
The atmospheric pressure is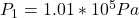
The temperature is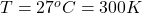
Volume at the end of the stroke is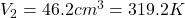
The increased pressure is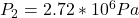
Now to obtain the temperature we are going to apply the ideal gas equation to this question and this is mathematically given as
Where P is the pressure , V sis the volume T is the temperature
While is the rate constant and n is the number of mole which is constant in this question hence
=>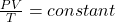
Making the subject of the formula we have
the subject of the formula we have
Therefore