Share
A chamber fitted with a piston can be controlled to keep the pressure in the chamber constant as the piston moves up and down to increase or
Question
A chamber fitted with a piston can be controlled to keep the pressure in the chamber constant as the piston moves up and down to increase or decrease the chamber volume. The chamber contains an ideal gas at 296 K and 1.00 atm.
What is the work done on the gas as the piston compresses it from 1.00 L to 0.633 L?
Express your answer with the appropriate units.
W =
J
in progress
0
Physics
3 years
2021-08-16T04:04:17+00:00
2021-08-16T04:04:17+00:00 1 Answers
18 views
0

Answers ( )
Answer:
Explanation:
Given:
isobaric process of a piston cylinder
pressure of the gas in the chamber,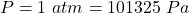
temperature of the gas in the chamber,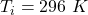
initial volume of the gas,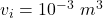
final volume of the gas,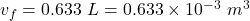
Now the work done is given as: