Share
A recent study found that electrons that have energies between 3.45 eV and 19.9 eV can cause breaks in a DNA molecule even though they do no
Question
A recent study found that electrons that have energies between 3.45 eV and 19.9 eV can cause breaks in a DNA molecule even though they do not ionize the molecule. If a single photon were to transfer its energy to a single electron, what range of light wavelengths could cause DNA breaks?minimum wavelength?
maximum wavelength?
in progress
0
Physics
4 years
2021-08-06T00:45:27+00:00
2021-08-06T00:45:27+00:00 1 Answers
40 views
0

Answers ( )
Answer:
The Minimum wavelength is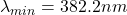
The Maximum wavelength is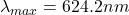
Explanation:
From the question we are told that
The energy range is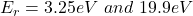
Considering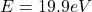
When a single photon is transferred to to an electron the energy obtained can be calculated as follows
This energy is mathematically represented as
Here h is the Planck’s constant with value of
c is the speed of light with value of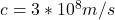
Substituting values and making the subject of the formula
the subject of the formula
Considering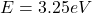
When a single photon is transferred to to an electron the energy obtained can be calculated as follows
This energy is mathematically represented as
Substituting values and making the subject of the formula
the subject of the formula