Share
The Bohr radius a0 is the most probable distance between the proton and the electron in the Hydrogen atom, when the Hydrogen atom is in the
Question
The Bohr radius a0 is the most probable distance between the proton and the electron in the Hydrogen atom, when the Hydrogen atom is in the ground state. The value of the Bohr Radius is: 1 a0 = 0.529 angstrom. One angstrom is 10-10 m. What is the magnitude of the electric force between a proton and an electron when they are at a distance of 2.63 Bohr radius away from each other?
in progress
0
Physics
4 years
2021-08-27T07:02:44+00:00
2021-08-27T07:02:44+00:00 1 Answers
57 views
0

Answers ( )
Answer:
The electric force is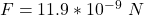
Explanation:
From the question we are told that
The Bohr radius at ground state is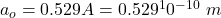
The values of the distance between the proton and an electron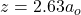
The electric force is mathematically represented as
Where n and p are charges on a single electron and on a single proton which is mathematically represented as
and k is the coulomb’s constant with a value
substituting values