Share
Un átomo Y tiene 13 protones y 13 electrones y pierde 3 electrones, represente el ion
Question
Lost your password? Please enter your email address. You will receive a link and will create a new password via email.
Answers ( )
Answer:
Explanation:
Notice that in the periodic table, the atom with 13 protons is the aluminum, whose chemical symbol is;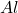 .
.
When it is neutral (same number of protons than orbital electrons) there is nothing particular added to its symbol, but when it has lost 3 electrons, now the charges of the nucleus compared to such of the orbits are not in balance any more, because there are three positive charges unpaired. Now it is an ion, and the way to represent it is to add on the upper right of its symbol, a a “3+” representing the charge imbalance: