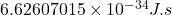Share
Science If you want to find the energy quantum of light, you multiply the frequency of the radiation (v) by “h”. What is “h”?
Question
Lost your password? Please enter your email address. You will receive a link and will create a new password via email.
Answers ( )
Answer:
“h” signifies Planck’s constant
Explanation:
In the equation energy E = h X v
The “h” there signifies Planck’s constant
Planck’s constant is a value, that shows the rate at which the energy of a photon increases/decreases, as the frequency of its electromagnetic wave changes.
It was named after Max Planck who discovered this unique relationship between the energy of a light wave and its frequency.
Planck’s constant, “h” is usually expressed in Joules second
Planck’s constant =