Share
To make hot chocolate for you and your friends, you must melt 3.0 kg of snow (0.0°C) and heat it to 70.0°C to make the hot chocolate. Calcul
Question
To make hot chocolate for you and your friends, you must melt 3.0 kg of snow (0.0°C) and heat it to 70.0°C to make the hot chocolate. Calculate the total amount of heat needed.
Heat of fusion of ice = 3.34 × 10^5 J/kg, Specific Heat of Water = 4180 J/(kg.K)
in progress
0
Physics
4 years
2021-08-02T07:44:24+00:00
2021-08-02T07:44:24+00:00 1 Answers
9 views
0

Answers ( )
Answer: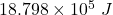
Explanation:
Given
Mass of snow is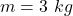
First snow is converted to water at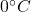 and then it is taken to
and then it is taken to 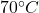
Heat required to convert the ice into water at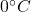
Heat required to raise temperature from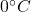 to
to 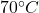
Total heat required