Share
Iron is obtained by reaction of hematite (Fe2O3) with carbon monoxide. From the balanced reaction below, how many grams of iron is produced
Question
Iron is obtained by reaction of hematite (Fe2O3) with carbon monoxide. From the balanced reaction below, how many grams of iron is produced when 24.6 mol of hematite is used up in the reaction?
Fe2O3 + 3CO ® 2Fe + 3CO2
in progress
0
Chemistry
4 years
2021-09-05T08:00:20+00:00
2021-09-05T08:00:20+00:00 1 Answers
15 views
0

Answers ( )
Answer: 2755.2 g of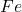 will be produced from 24.6 mol of haematite.
will be produced from 24.6 mol of haematite.
Explanation:
The balanced chemical equation is:
According to stoichiometry :
1 mole of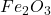 produce = 2 moles of
produce = 2 moles of 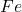
Thus 24.6 moles of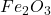 will produce=
will produce= of
of 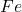
Mass of
Thus 2755.2 g of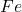 will be produced from 24.6 mol of haematite.
will be produced from 24.6 mol of haematite.