Share
Consider the oxidation of sodium metal to sodium oxide described by the balanced equation: 4 Na + O2 → 2 Na2O. What is the theoretical
Question
Lost your password? Please enter your email address. You will receive a link and will create a new password via email.
Answers ( )
Answer:
1116g
Explanation:
We’ll convert moles O2 -> moles of Na2O -> grams of Na2O.
Based on our balanced equation, we have 1 mole of O2 for every 2 moles of Na2O. This is our mole to mole ratio.
9 mol O2 x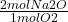 = 18 mol Na2O
= 18 mol Na2O
We can convert mols -> grams using the molar mass of Na2O- 62g.
18 mol Na2O x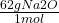 = 1116g
= 1116g