Share
A cylinder of Krypton has contains 17 L of Ar at 22.8 atm and 112 degrees celsisus. How many moles are in the cylinder?
Question
Lost your password? Please enter your email address. You will receive a link and will create a new password via email.
Answers ( )
Given :
A cylinder of Krypton has contains 17 L of Ar at 22.8 atm and 112 degrees Celsius.
To Find :
How many moles are in the cylinder.
Solution :
We know, by ideal gas equation :
Here, R is gas constant and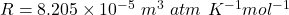
Converting all given in required units and putting in above equation, we get :
Hence, this is the required solution.