A chemical equation which contains same number of atoms on both reactant and product side is called a balanced chemical equation.
For example,
Here, number of atoms on reactant side are as follows.
V = 2
O = 5
Ca = 1
The number of atoms on product side are as follows.
V = 1
O = 1
Ca = 1
In order to balance this equation, multiply Ca by 5 on reactant side. Multiply V by 2 and CaO by 5 on product side. Therefore, the equation can be rewritten as follows.
Now here, number of atoms on reactant side are as follows.
V = 2
O = 5
Ca = 5
The number of atoms on product side are as follows.
V = 2
O = 5
Ca = 5
Since, there are same number of atoms on both reactant and product side. So, the equation is now balanced.
Thus, we can conclude that the balanced equation is .
Answers ( )
Answer: The balanced equation is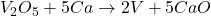 .
.
Explanation:
A chemical equation which contains same number of atoms on both reactant and product side is called a balanced chemical equation.
For example,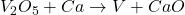
Here, number of atoms on reactant side are as follows.
The number of atoms on product side are as follows.
In order to balance this equation, multiply Ca by 5 on reactant side. Multiply V by 2 and CaO by 5 on product side. Therefore, the equation can be rewritten as follows.
Now here, number of atoms on reactant side are as follows.
The number of atoms on product side are as follows.
Since, there are same number of atoms on both reactant and product side. So, the equation is now balanced.
Thus, we can conclude that the balanced equation is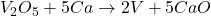 .
.