What happens when 10g of nitrogen combines with 10 grams of hydrogen Question What happens when 10g of nitrogen combines with 10 grams of hydrogen in progress 0 Chemistry Thông Đạt 4 years 2021-07-30T10:40:25+00:00 2021-07-30T10:40:25+00:00 1 Answers 11 views 0
Answers ( )
It would create 12.1 g of ammonia (NH3).
The amount of hydrogen that is reacted in this reaction is:
(10 g N2)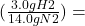 2 g H2
2 g H2
According to the Law of Conservation of Mass, the mass of ammonia produced is the combination of hydrogen and nitrogen reacted or:
2.1g + 10.0g = 12.1 g of NH3