Share
Phosphine, PH3, a reactive and poisonous compound, reacts with oxygen as follows: 4PH3(g) 8O2(g) – P4O10(s) 6H2O(g) If you need to make
Question
Phosphine, PH3, a reactive and poisonous compound, reacts with oxygen as follows: 4PH3(g) 8O2(g) – P4O10(s) 6H2O(g) If you need to make 6.5 moles of P4O10, how many moles of PH3 is required for the reaction
in progress
0
Chemistry
3 years
2021-07-16T22:01:49+00:00
2021-07-16T22:01:49+00:00 1 Answers
90 views
0

Answers ( )
Answer: 26 moles of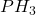 are required for the reaction.
are required for the reaction.
Explanation:
We are given:
Moles of = 6.5 moles
= 6.5 moles
The given chemical reaction follows:
By the stoichiometry of the reaction:
If 1 mole of is produced by 4 moles of
is produced by 4 moles of 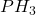
So, 6.5 moles of will be produced by =
will be produced by = 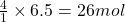 of
of 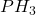
Hence, 26 moles of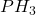 are required for the reaction.
are required for the reaction.