Share
When heat is applied to 80 grams of CaCO3, it yields 39 grams of CO2. Determine the percentage of the yield.
Question
Lost your password? Please enter your email address. You will receive a link and will create a new password via email.
Answers ( )
Answer: The percentage yield of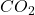 is 90.26%.
is 90.26%.
Explanation:
The number of moles is defined as the ratio of the mass of a substance to its molar mass. The equation used is:
Given mass of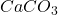 = 80 g
= 80 g
Molar mass of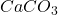 = 100 g/mol
= 100 g/mol
Plugging values in equation 1:
For the given chemical equation:
By the stoichiometry of the reaction:
If 1 mole of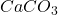 produces 1 mole of
produces 1 mole of 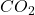
So, 0.8 moles of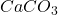 will produce =
will produce = 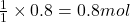 of
of 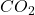
Molar mass of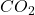 = 44 g/mol
= 44 g/mol
Plugging values in equation 1:
The percent yield of a reaction is calculated by using an equation:
Given values:
Actual value of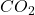 = 35.2 g
= 35.2 g
Theoretical value of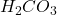 = 39 g
= 39 g
Plugging values in equation 2:
Hence, the percentage yield of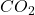 is 90.26%.
is 90.26%.