Share
I want to create water out of 45.4 Liters of Oxygen at STP. How much water will I produce? STP: Standard Temperature and Pressure
Question
Lost your password? Please enter your email address. You will receive a link and will create a new password via email.
Answers ( )
Answer:
72.96 of water produce by 45.4 L of oxygen at STP.
Explanation:
1 mole of oxygen=22.4 L at STP
11.2 L of oxygen required to produce water=1 mole
1 L of oxygen required to produce water=1/11.2 mole
45.4 L of oxygen required to produce water=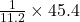
45.4 L of oxygen required to produce water=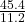 moles
moles
1 mole of water=18 g
Hence, 72.96 of water produce by 45.4 L of oxygen at STP.