Share
8. How many calories of heat energy (Q) would be released as a 2,500 g iron (Fe) frying pan at 190˚C cools to 25˚C?
Question
Lost your password? Please enter your email address. You will receive a link and will create a new password via email.
Answers ( )
Answer:
Explanation:
From the question we are told that:
Mass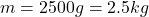
Initial heat of pan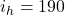
Final heat of pan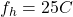
Generally the equation for Heat energy released is mathematically given by