Share
3. Given the following equation: 2 K+ Cl2 =>2 KCI How many grams of KCl are produced from 2.50 g of K and excess Cl2?
Question
Lost your password? Please enter your email address. You will receive a link and will create a new password via email.
Answers ( )
Answer:
4.767 grams of KCl are produced from 2.50 g of K and excess Cl2
Explanation:
The balanced equation is
2 K+ Cl2 —>2 KCI
Here the limiting agent is K. Hence, the amount of KCl will be calculated as per the mass of 2.50 gram of K
Mass of one atom/mole of potassium is 39.098 grams
Number of moles is 2.5 grams =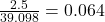
So, 2 moles of K produces 2 moles of KCL
0.064 moles of K will produces 0.064 moles of KCl
Mass of one molecule of KCl is 74.5513 g/mol
Mass of 0.064 moles of KCl is 4.767 grams