Share
25. How many ml of a .0023M strontium hydroxide solution are needed to completely react 15.0 mL of 0.012M hydrochloric acid?
Question
Lost your password? Please enter your email address. You will receive a link and will create a new password via email.
Answers ( )
Answer: There are 78.26 mL of a 0.0023M strontium hydroxide solution are needed to completely react 15.0 mL of 0.012M hydrochloric acid.
Explanation:
Given: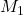 = 0.0023 M,
= 0.0023 M,  = ?
= ?
Formula used to calculate volume of strontium hydroxide solution is as follows.
Substitute the values into above formula as follows.
Thus, we can conclude that there are 78.26 mL of a 0.0023M strontium hydroxide solution are needed to completely react 15.0 mL of 0.012M hydrochloric acid.