Share
2. If a gas at 500 mL has a temperature of 45°C, then what is the new volume when the temperature is increased to 65°C? Show your work.
Question
Lost your password? Please enter your email address. You will receive a link and will create a new password via email.
Answers ( )
Answer:
V2= 531.4 ml
Explanation:
T1= 273+45 °C= 318 Kelvin
T2= 273+65 °C= 338 Kelvin
V1= 500ml *( 0.001L/1ml)= 0.5 Liters
V2=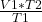 =
= 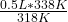 = 0.5314465409 Liters* (1 ml/0.001 L)= 531.4 ml
= 0.5314465409 Liters* (1 ml/0.001 L)= 531.4 ml