Share
You heat a 5.05 g piece of titanium to 98.2 oC and place it into 20.00 mL of room temperature water (24.8 oC ). The temperature of the water
Question
You heat a 5.05 g piece of titanium to 98.2 oC and place it into 20.00 mL of room temperature water (24.8 oC ). The temperature of the water rises to 27.3 oC. The specific heat of water is 4.184 J/goC. The density of water is 0.997 g/mL. A. How much heat is absorbed by the water (in units of J)
in progress
0
Chemistry
4 years
2021-07-13T13:13:49+00:00
2021-07-13T13:13:49+00:00 1 Answers
17 views
0

Answers ( )
Answer: The heat absorbed by the water is 52.823 J.
Explanation:
Given: Mass of metal = 5.05 g
Specific heat of water = 4.184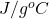
Initial temperature =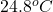
Final temperature =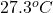
Formula used to calculate heat absorbed is as follows.
where,
q = heat
m = mass of substance
Substitute the values into above formula as follows.
Thus, we can conclude that heat absorbed by the water is 52.823 J.